Abstract
Introduction: Voxelotor is a small molecule FDA approved for the treatment of sickle cell disease (SCD) which has been proved to reduce hemolysis and increase hemoglobin (Hb) levels. Voxelotor binds to the alpha subunit of Hb and prevents accurate quantification of Hb subtypes (HbA, HbS, and HbF) by high-performance liquid chromatography (HPLC) and capillary zone electrophoresis (CZE), two commonly used methods of Hb fractionation measurement. Both methods display characteristic peaks that identify the presence of different Hb types, and some of these peaks split in the presence of voxelotor. There is reported variability in the hematologic response to voxelotor therapy, defined as a 1g/dL increase in Hb. A lack of response may be attributed to poor compliance or absorption. Given that the only method for measuring concentration of drug is a liquid chromatograph tandem-mass spectrometry assay (LC-MS/MS) performed at bioanalytical laboratories, there is an unmet need to develop methods to assess drug concentrations that are more accessible to ensure optimal therapeutic dosing in patients who may have a perceived lack of response. The goal of this work was to derive an equation based upon the degree of peak splitting observed in Hb fractionation measurements to allow for an estimation of whole blood drug concentrations.
Methods: To generate an equation to estimate voxelotor concentration by degree of peak splitting, blood samples were spiked with known concentrations of voxelotor from 0 to 600 µmol/L and CZE and HPLC were performed. Samples had Hb variants quantified by CZE using the Capillarys 2 FlexPiercing Instrument running the Hemoglobin (E) program (Sebia, Georgia) and HPLC using BioRad Variant II instrument running the β-thalassemia Short Program (BioRad, California). Equations were derived using a regression algorithm.
To validate the equations, patients with HbSS/HbSβ0 who had decided with their clinician to start voxelotor treatment were enrolled in an IRB approved protocol. Voxelotor was initiated at 1500 mg daily and samples were taken at day 0 (pre-dose), 14, 30, and 60 days. CZE and HPLC, CBC with retic, LDH, BMP and liver tests were performed. To determine blood concentration, samples were sent to Worldwide Clinical Trials where a validated LC-MS/MS method was performed. Whole blood concentration was estimated based on the derived equations for CZE and HPLC and estimates were compared to the measured concentrations. Comparison was done using a general linear regression model adjusting for random effects between individuals.
Results: 21 patients were enrolled and completed the study (100% HbSS/Hbβ0, 57% on hydroxyurea, median age 35.9 (interquartile range 30.2-37.5). Based on in vitro data, the following equations were derived:
CZE: [voxelotor] (μmol/L) = -99.1272 + 7.097756 * F% + 12.52202 * %VarA2
'F%' is the value of Hb F as directly reported by the CZE, '%VarA2' is the percent of A2 bound to voxelotor as determined by inspection of the split peak.
HPLC: [voxelotor] (umol/L) = 3.968591+6.405132 * F% +10.96542* %VarS
'F%' is the value of Hb F as directly reported from the instrument, '%VarS' is the percent of S bound to voxelotor as determined by inspection of the split peak.
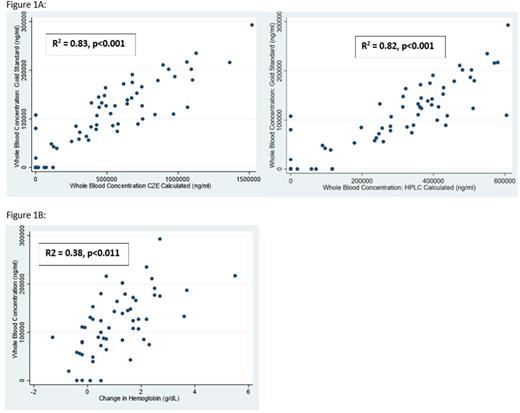
Calculated results using CZE and HPLC were shown to correlate strongly with the gold standard concentration testing (CZE: R2 = 0.83, p<0.001, HPLC: R2=0.82, p<0.001). Figure 1 Whole blood concentration had a weak but significant correlation with Hb increase at all timepoints (R2=.38, p<0.011). An increase in Hb of >1g/dL was seen in 24/34 (71%) of patients with a whole blood concentration > 100,000 ng/ml but only 4/22 (18%) of those with a concentration of <100,000 ng/ml (p<0.001).
Conclusions: We have demonstrated that equations can estimate whole blood concentration of voxelotor when using two easily accessible tests: CZE and HPLC. This data may allow clinicians to assess and potentially optimize response to therapy in the absence of access to LC-MS/MS. Higher blood concentrations were associated with better responses, suggesting compliance and/or drug absorption and distribution may play a role in response. Further studies should be done to evaluate the minority of patients who demonstrate measurable levels of voxelotor via whole blood concentration but do not demonstrate a hematologic response. Ongoing studies are evaluating the relationship between these equations and percent voxelotor-hemoglobin adduct.
Disclosures
Curtis:Global Blood Therapeutics: Consultancy, Honoraria; Novartis: Honoraria. Minniti:Pfizer Inc: Research Funding. Ngyuen Dang:Global Blood Therapeutics: Current Employment. Pochron:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Campbell:Global Blood Therapeutics: Research Funding; Sebia, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal